SAFETY BELT WITH DOUBLE D-RING
August 27, 2021
AIKO HEAD RECHARGEABLE TORCH LIGHT HEAD MOUNTED LED
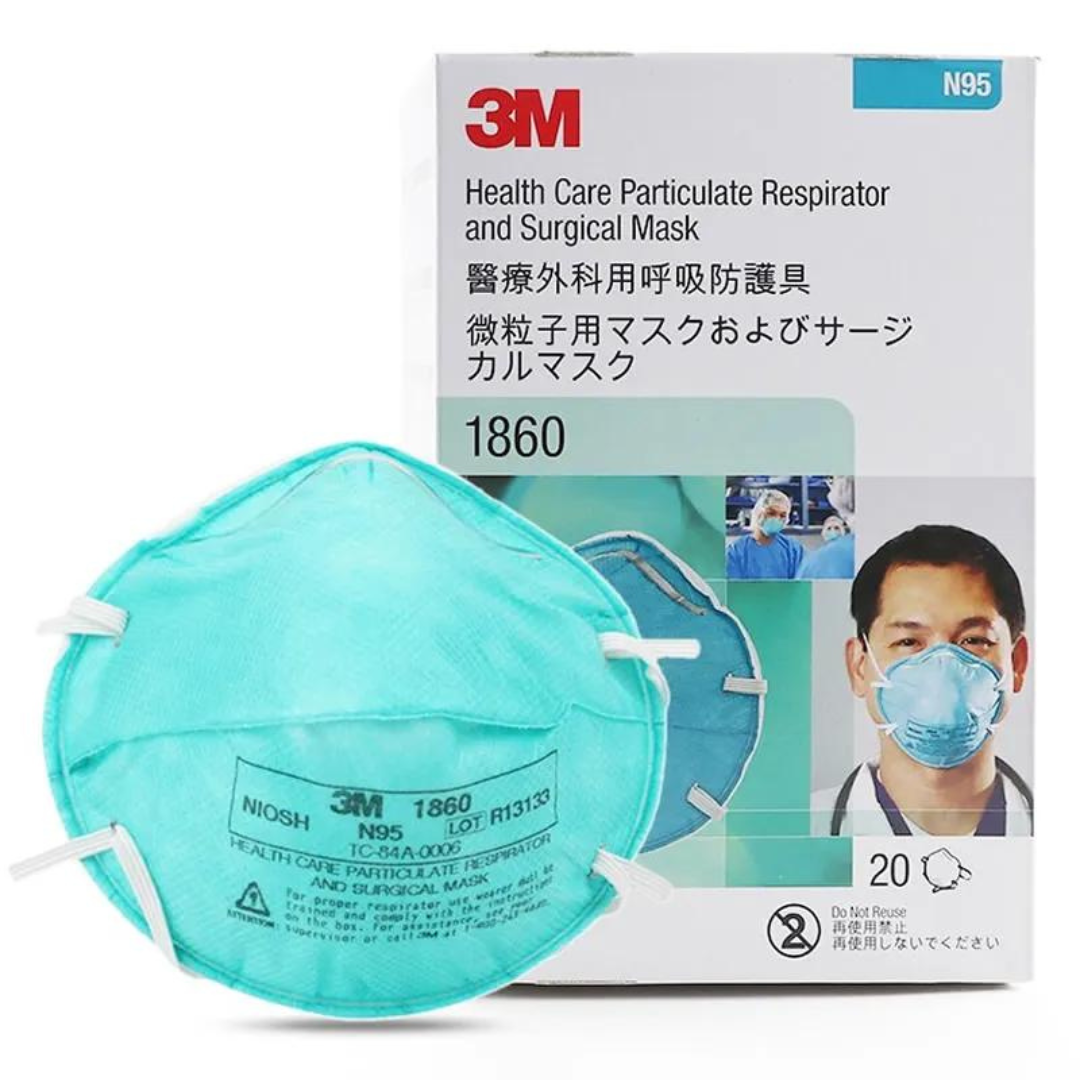
September 17, 20213M™ Particulate Respirator 1860, N95 Mask
රු200.00
• Intended to be worn by operating room personnel during
surgical procedures to help protect both the surgical
patient and the operating room personnel from transfer of
microorganisms, body fluids, and particulate material.
• Always follow User Instructions and use in manners as
indicated
Do Not Use for
• DO NOT use in industrial settings
• DO NOT use for gases or vapors (i.e. anesthetic gases
such as isoflurane or vapors from sterilant such as
glutaraldehyde.)
• DO NOT use in any manner not indicated in the User
Instructions
Approvals and Standards
• Meets NIOSH 42 CFR 84 N95 requirements for a
minimum 95% filtration efficiency against solid and liquid
aerosols that do not contain oil.
• NIOSH approval number: TC-84A-0006
• FDA cleared for use as a surgical mask
• Health Canada Class I medical device
• Bacterial Filtration Efficiency F2101 >99% BFE
• OSHA Assigned Protection Factor (APF) 10
• Australia TGA approved
• NIOSH approved N95 rating
• FDA cleared for use as a surgical mask
• Fluid Resistance (1860 at 120mm Hg & 1860S at 80 mmHg)
• Flammability Rating Class I
• Adjustable nose clip
• Braided and stapled headbands
• Particulate Respirator and Surgical Mask
- NIOSH approved for at least 95 percent filtration efficiency against aerosols free of oil; time use restrictions may apply.
The 1860 and 1860S healthcare respirators are designed to help provide respiratory protection for the wearer against certain airborne particles. They meet CDC guidelines for Mycobacterium tuberculosis exposure control. As disposable particulate respirators, they are intended to reduce wearer exposure to certain airborne particles including those generated by electrocautery, laser surgery, and other powered medical instruments. As surgical masks, they are designed to be fluid resistant to splash and spatter of blood and other infectious materials. The 1860 and 1860S particulate respirators are personal protective equipment (PPE) that are primarily intended to
protect the health care workers by reducing exposure to harmful airborne particles which are small enough to be inhaled – typically particles less than 100 microns in size. These include airborne particles that may contain biological material e.g. Bacillus anthracis, Mycobacterium tuberculosis, mould and the virus that causes Severe Acute Respiratory Syndrome (SARS), and Influenza.
Related products
-
CAMPING SLEEPING BAG
රු6,800.00